
An example is gluconic acid (derived from glucose)Ģ.

This oxidation can be accomplished by bromine. The oxidation of aldoses can lead to the formation of three different kinds of carboxylic acids.ġ) Aldonic acids in which the aldehydic group in C1 is oxidized to COOH. Inositols (myo-inositol and scyllo-inositol) are cyclic hexitols. Some of these are natural compounds, e.g., mannitol, glucitol (or sorbitol) and ribitol. sodium borohydride) allows to reduce the carbonyl group to alcohol, the resulting compounds (polyhydric alcohols) having the general formula HOCH 2 nCH 2OH are known as alditols ( glycitols is an obsolescent synonym for alditols) and named pentitols, hexitols, etc. Reduction of sugars with a mild reducing agents (e.g.
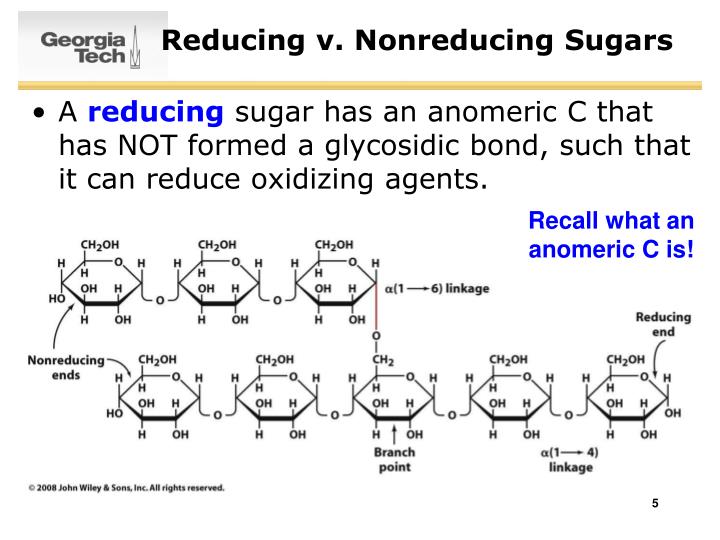
#Non reducing anomeric carbon free#
However when the anomeric carbon is involved in a chemical bond no free carbonyl group can exist and no reduction of the reagents above can be observed and the sugars are referred to as non-reducing sugars. Most of monosaccharides and disaccharides are reducing sugars. The Tollens' reagent: silver ion in ammonia solution. In the presence of reducing sugars Cu 2+ is reduced to Cu + which precipitates as Cu 2O (red) and the blue colour of the Fehling solution (due to the complex tartrate-Cu 2+) disappears. The reactive of Fehling is an alkaline, blue solution of Cu 2+ complexed with tartrate. For example, they are able to reduce the reactive of Fehling or the Tollens' reagent and are generally referred to as reducing sugars. When the equilibrium is reached we can measure the same final value obtained forthe alpha anomer (52.5°).Īs said above, the open and cyclic structures readily interconvert and a significant amount of sugar exists in the carbonyl form and thus they undergo to typical reactions of the carbonyl group. Conversely the solution of the beta anomer has an initial specific optical rotation of 19°. This phenomenon, known as mutarotation, is due to the interconversion of the differents forms until an equilibrium is reached which corresponds to 64% of beta anomer and 36 of the alpha. When crystalls of alpha-D-glucose are dissolved in water we can observe that initially the specific optical rotation is 112° and decreases with the time until to reach a value of 52.5°.
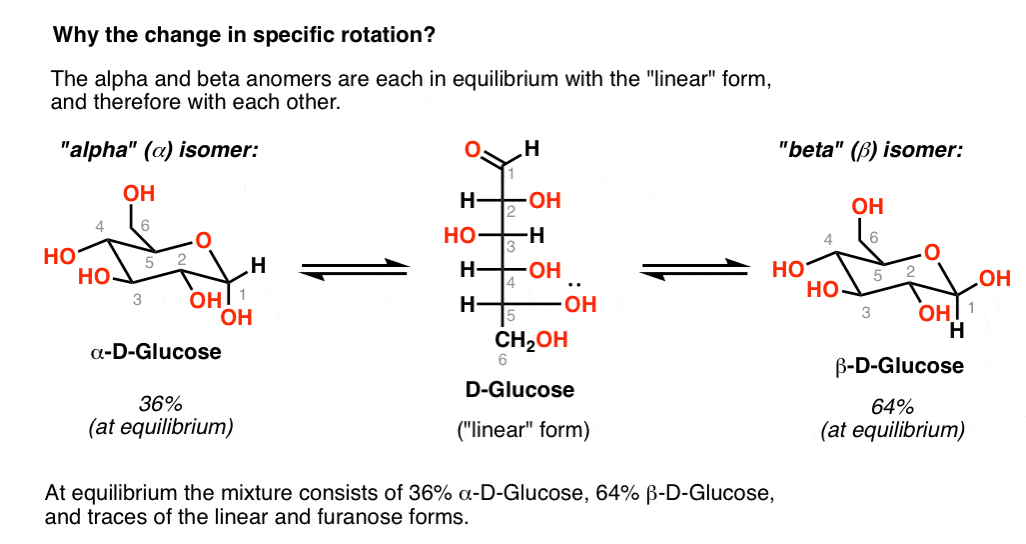
In solutions, the open and cyclic structures readily interconvert and the term glucose (fructose) is used to refers to the mixture of the differents forms.

In the case of fructose the forms alpha and beta refers to the position of the hydroxyl group bound to anomeric carbon at position 2. The carbon in position 1 is named anomeric and alpha and beta forms are said anomers. After the formation of the cycle the carbon in position 1 becomes asymmetric and the OH group can be below the plane of the ring (alpha-form) or above the plane of the ring (beta-form). The cycle is planar and should be imagined to be perpendicular to the screen with the bolded line being the closest to the observeur. Taking as an example glucopyranose we illustrate the main feature of the formula. The cyclic structures given above for glucose and fructose are known as projections of Haworth. The cycle for its resemblance with furan is named fructofuranose. In the case of fructose the the hydroxyl group in position 5 reacts with the ketonic group to give an intramolekular hemiketals. The cycle for its resemblance with pyran is named glucopyranose. In solution, the predominant structure of glucose is a cycle arising from the reaction of the hydroxyl group in position 5 with the aldehydic group (an intramolecular hemiacetals). Also in this case the prefix D refers to the configuration of the asymmetric carbon atom farthermost from the aldehydic or ketonic group. Two common hexoses (6 carbon atoms) are D-glucose and D-fructose. Two common tetrose are D-erytrose and D-threose, the prefix D means that the configuration of the asymmetric carbon atom farthermost from the aldehydic or ketonic group is the same as D-glyceraldehyde. Glyceraldehyde has an asymmetric carbon atom and thus two steroisomers (D-Glyceraldehyde and L-Glyceraldehyde) exist. The most simple carbohydrates are glyceraldehyde and dihydroxyacetone. They are generally indicated, depending on the number of carbon atoms, as thrioses, tetroses, pentoses, hexoses, etc. Monosaccharides is a general term indicating polyhydroxyaldehydes (aldoses) and polyhydroxyketones (ketoses) with at least two hydroxyl groups.


 0 kommentar(er)
0 kommentar(er)
